Enhanced coagulation of surface waters with high organic con
| 论文类型 | 技术与工程 | 发表日期 | 2005-06-01 |
| 作者 | 马军,G.B.,Li,Z.L.,Chen | ||
| 摘要 | J. Ma, G.B. Li, Z.L. Chen, G.R. Xu, G.Q. Cai* School of Municipal and Environmental Engineering, Harbin University of Architecture and Engineering, POBox 627, Harbin 150090, PR China,*Heilongjiang Electrical Power Compan | ||
J. Ma, G.B. Li, Z.L. Chen, G.R. Xu, G.Q. Cai*
School of Municipal and Environmental Engineering, Harbin University of Architecture and Engineering, POBox 627, Harbin 150090, PR China,*Heilongjiang Electrical Power Company, Harbin 15001, PR China
Abstract Laboratory and full-scale experiments were conducted for enhancing the coagulation of stabilised surface waters with high organic content by permanganate preoxidation. It was indicated that permanganate preoxidation enhanced both the processes of coagulation and filtration of selected surface waters, River Songhua water, Qitaihe Reservoir water and Ba Reservoir water. Permanganate preoxidation is especially effective for enhancing the coagulation of very stable surface waters with relatively high organic content. Experimental results showed that the manganese dioxide produced in situ during permanganate preoxidation played an important role in enhancing the coagulation and filtration processes. The experimental results suggested that manganese dioxide may adsorb naturally occurring organic materials through surface bonding to form bigger particulates, thus increasing the floc density and improving the removal of organic particulates and possibly the inorganic fine particles. Manganese dioxide was shown to adsorb humic acid
and enhance its removal by filtration.
Keywords Enhanced coagulation; flocculation; oxidation; manganese dioxide; permanganate; preoxidation.
Introduction
Particle stabilisation by NOM
The conventional water treatment process, coagulation-sedimentation-filtrationdisinfection,has been used as the principal process in China for many years. However,because of the earth erosion and water pollution problems, there is a tendency towards increase in turbidity and organic content in raw waters. This has caused some difficulty for water treatment.
Coagulation plays an important part in the conventional water treatment process.Traditionally, the coagulation process is described in terms of the destabilisation of colloids initially present in a water supply. These colloids may include organic and inorganic particulates. Recent investigations showed that naturally occurring organic materials (NOM), particularly the pool of dissolved organic carbon, caused strong stabilisation of inorganic particulates in water. It was reported (O’Melia, 1987) that in the presence of naturally occurring organic materials the coagulation kinetics of inorganic particulates mainly depended on the characteristics and the concentration of natural organics, rather than inorganic particulates themselves. Jekel (1986) reported that adsorbed with humic acid on the surface, SiO2 particles increased the stability one-fold, or the collision efficiency decreased one-fold; the stability is further increased with the increase of humic acid concentration and with the decrease of pH. It was believed that this phenomenon was due to the surface hydrogen bonding of nondissociated acidic groups by the humic acids on SiO2, whilst Gibbs (1983) believed that the increased stability of inorganic colloids by organic materials was due to the organic coating formed on the surface of inorganic colloids, causing steric hindrance and repulsive action between the colloidal particles, in which the organic material may act as dispersion agents, among inorganic particulates. The much higher surface charge on naturally occurring organics than on clay particles is believed to be the principal reason for the stabilisation of inorganic colloids by naturally occurring materials (Edzwald,1993).
As a result of the stabilisation effect, the flocculant dosages are much increased in the case of high organic content. Narkis and Rebhun (1975) dispersed the clay particles or clays coated with naturally occurring organic materials into humic and fulvic acid solutions, then
coagulated with positively charged polyelectrolyte. It was found that the polyelectrolyte reacted firstly with the humic and fulvic acids, only after polyelectrolyte dosages were increased high enough to neutralise the negative charges on the surface of humic and fulvic acids did polyelectrolyte exhibit a bridging effect between the particulates. Yao and Yan (1989) observed that when the fulvic acid concentration in the clay suspension was increased by 3 mg/L (as TOC), the alum dosage needed was increased to 5.3 times to destabilise the suspension; and if the increase of fulvic acid concentration was 7 mg/L (as TOC), the alum dosages had to be 10.2 times as much to cause the stabilisation. This experimental result is close to that assumed by Edzwald (1993) based on the comparison of surface charges of fulvic acid and clays in the water. It was also shown (Edzwald, 1993) that the turbidity (30 NTU), low alkalinity (<50 mg/L/CaCO3), but high DOC content (20 mg/L) was several times higher than the case of a surface water with high turbidity (670 NTU), but moderate hardness (150 mg/L CaCO3), and low DOC content (3 mg/L). These experimental results clearly showed that the presence of organic materials is one key factor affecting the destabilisation of clay particles in waters.
Preoxidation
Prechlorination. One of the principal methods for improving the coagulation and filtration processes is preoxidation. It is generally aimed at destroying the organic coating on the surface of particles, or destabilisation effect of algae species on the colloidal particles. The
earliest use of preoxidation to aid the coagulation may be the prechlorination process. It was effective in many cases where the organic or ammonia contents were relatively high. However, prechlorination leads to the formation of a range of harmful chlorination byproducts, and it is progressively restricted in most countries.
Preozonation. To find out an alternative method of preoxidation, much of the recent work has been conducted to evaluate ozone preoxidation as an aid to coagulation, flocculation, and filtration. It has been shown that at very low ozone dosages preozonation improved the removal of turbidity by coagulation (Jekel, 1983). Preozonation at a dosage of 1.8 mg/L was shown to reduce the residual turbidity in an in-line direct filtration process (Petrusevski et al., 1995). Edwards et al. (1993) expected the preozonation would have an adverse effect on organic matter removal at ozone doses greater than 0.7 mg per mg TOC, but enhanced removal could occur if relatively high concentrations of volatile organic matter were present; preozonation also hindered the removal of turbidity and increased the concentration of residual coagulant metals at low coagulant doses. It was reported (Chang and Singer, 1991) that the optimal effect for destabilising suspensions was obtained with an ozone dose of 0.4-0.8 mg/L for hardness to TOC ratios exceeding 25. Generally, preoxidation has been reported to either hinder or has no effect on DOC removal by coagulation (Reckhow and Singer, 1984; Edwards and Benjamin, 1991; Edwards et al., 1993), but the
nature of DOC was shown to have changed due to the clear decrease of the colour and ultraviolet absorbance (Chang and Singer, 1991). However, Tobiason et al. (1993) reported that preozonation caused a two-fold increase in raw water AOC and has little effect on raw water DOC. More coagulant was reported (Lefebvre, 1990) to achieve a maximum DOC removal at an ozone dose of 0.5 mg O3/mg-C in comparison to the case without preozonation. Becker and O’Melia (1995) reported that deterioration of DOC removal by preozonation was principally due to the formation of lower molecular weight organic materials. In contrast, preozonation was found to be more effective for biomass removal than prechlorination (Preis et al., 1991). It was also found (Petrusevski et al., 1995) that preozonation improved the particle removal efficiency in direct filtration and the optimal ozone dosage was 1.8 mg/L. It was reported that ozonation of waters containing bromide led to the formation of bromate and also enhanced the formation of brominated disinfection by-products, at a level suspected of being hazardous to health (Glaze et al., 1993).
Permanganate preoxidation. Permanganate has long been used to remove iron and manganese from ground water, to control taste and odour, in particular of algal origin. It has also been used as a disinfectant and an algicide. However, permanganate has received relatively little attention to date in comparison to ozone, partly because of its perceived relative inferior oxidation strength to ozone and also concern about residual manganese. Nevertheless, a few recent studies have indicated significant treatment improvements through the application of potassium permanganate. It was shown (Ma and Li, 1993; Ma et al., 1997) that permanganate preoxidation obviously enhanced the coagulation of several kinds of surface waters, with substantial reduction in the settled turbidity. The quality of filtered water was also substantially improved by permanganate preoxidation through a jar test (Ma and Li, 1993) and an in-line direct filtration process (Petrusevski et al., 1995). It was demonstrated that permanganate preoxidation reduced the THMFP for some surface waters (Singer et al.,
1980), for some typical THM precursors (Ma and Graham, 1996) and also reduced the formation of chlorophenols in the chlorine-disinfection process (Ma and Li, 1994).Permanganate preoxidation was shown to be effective for the removal of acrylamide, a byproduct resulting from the use of polyacrylamide (Ma et al., 1994a,b) and other organic pollutants (Ma and Li, 1991). Permanganate preoxidation was shown to have caused substantial reduction in mutagenic activities in filtered and disinfected waters (Li and Ma,1992).
In comparison to the existing special processes for treating waters with high organic content such as ozonation, ozone/activated carbon, activated carbon processes etc., which normally require high cost, permanganate preoxidation has the advantages of low cost, easy operation and maintenance. It may be an economic method to enhance the conventional water treatment process on occasions with limited funds for capital investment.
Objectives of the study. In this paper, the authors will further study the effectiveness of permanganate preoxidation in improving the coagulation of surface waters through laboratory and full-scale investigations.
Methods
Laboratory jar test
In a previous investigation (Ma and Li, 1993), it was found that permanganate preoxidation of a river water with turbidity around 200 NTU, Colour 20 NTU, pergamanate index 9-13 mg/L, caused parallel reduction in the residual turbidity of settled water at alum dosages between 20-70 mg/L, while for a water with very low turbidity and low organic content,permanganate preoxidation increased slightly the turbidity of the settled water though it reduced the filtered turbidity (Ma et al., 1997). This suggests that the effect of permanganate preoxidation may depend on the matrix of water to be treated. It is likely that permanganate oxidation is more effective under conditions of relatively high organic content and turbidity.
In order to have a deeper understanding of the mechanism of permanganate preoxidation to enhance the coagulation of surface waters, a laboratory study was further conducted with standard jar tests. Surface waters with high organic content were obtained either by spiking
humic acid into River Songhua water or from several real surface waters with high organic content (Qitaihe Reservoir water, Ba Reservoir water etc.). The experiment was carried out with a Six-Beaker Jar Test Apparatus. A carefully calculated amount of permanganate solution was injected into each beaker shortly (less than 5 min) before the addition of flocculant. After fast stirring for 0.5 min at 300 rpm, followed by slow stirring for 5 min at 35 rpm, the water was allowed to stand quiescently for 15 min. The supernatant was siphoned at 2 cm below the water surface, the residual turbidity and some other parameters were then determined. When necessary, the supernatant of each sample was further filtered through a filter paper (with pore size 2-4 μm) in order to evaluate the effect of permanganate preoxidation
on the quality of filtered water.
Full-scale investigation
Full scale investigations were conducted in several water treatment plants. Comparison tests were made with and without permanganate preoxidation. One of them is the Fourth Water Treatment Plant of Harbin, which uses River Songhua as its raw water. Normally the
turbidity of the raw water in the summer is much higher than in the winter. This plant used the conventional water treatment process (coagulation-sedimentation-filtration process), in which PAC was used as a flocculant. Having mixed with the flocculant, the water coagulated with a hydraulic flocculator and then settled in an inclined tube sedimentation tank, and filtered with no-valve hydraulic automatic filter, followed by chlorine-disinfection before entering into the distribution network. The output of this plant is 40 000 m3/d. In winter, River Songhua is covered with ice, thus the raw water quality is very stable. The second site of full-scale study is Qitaihe water treatment plant, which uses a reservoir as the source of its raw water. Since the reservoir receives the water from a large mountainous area, the humic acid content in the water is relatively high. The capacity of this water treatment plant is 5000 m3/d, which employs conventional water treatment processes. The third site of full scale study is the Ba reservoir water treatment plant, which uses Ba reservoir as the source of its raw water. Ba Reservoir is a shallow reservoir and contaminated by the municipal wastewater. The water treated by this plant was not for drinking purposes, only for industrial use. The capacity of this water treatment plant is 100 000m3/d. Typical raw water quality of these water treatment plants is shown in Table 1.
Experimental results
Laboratory study
Previous investigation (Ma and Li, 1993) showed that the optimum flocculant dosage to achieve the lowest residual turbidity was not changed after permanganate addition, though there was a consistent reduction in turbidity with the increase of permanganate dosages. It suggests that permanganate preoxidation will possibly not change substantially the surface charge of colloidal particles in water. It is possible that manganese dioxide produced in situ,which has strong adsorption properties, may adsorb the organic particulates through hydrogen bonding and play a bridging role between the particulates.
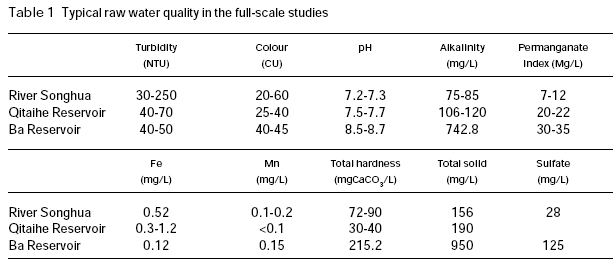
Figure 1 shows the settled and filtered turbidity with respect to permanganate dose in the coagulation of River Songhua water (raw water turbidity 237 NTU; pH 7.2; permanganate index 11 mg/L). This raw water is characterised by relatively high turbidity and organic content. It is shown that permanganate preoxidation caused near linear reduction in the settled turbidity as permanganate dosage increased up to 1.5 mg/L. Higher dose of permanganate did not achieve further reduction in turbidity. In the case of filtered water, great reduction in turbidity was observed at low permanganate dosages (≤1.0 mg/L), and further increasing permanganate dose up to 4 mg/L achieved slightly additional reduction in turbidity. The results of this study indicated that permanganate preoxidation is effective in enhancing the coagulation of this surface water.
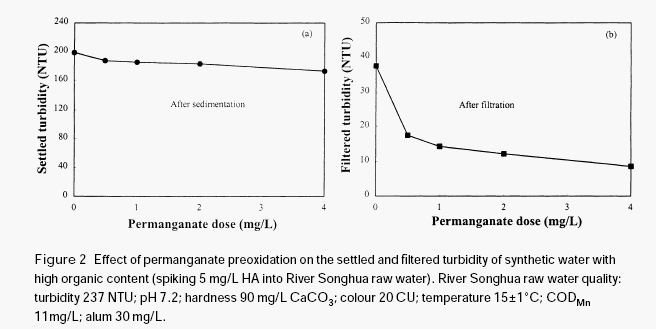
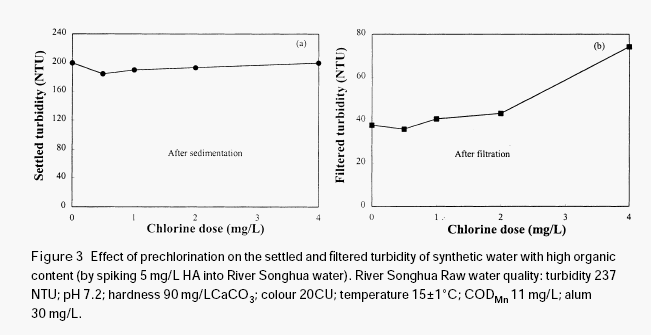
The effectiveness of permanganate preoxidation to aid the coagulation of surface waters depends on many factors and the mechanics may be very complex. However, it is expected that the manganese dioxide produced in situ during permanganate preoxidation may have a very important role in enhancing the coagulation and the filtration of surface water, even if some other mechanisms such as destroying organic coating on the surface of inorganic colloidal particles by oxidation may exist in the process of permanganate preoxidation. Since the newly formed manganese dioxide has very strong adsorption ability, it may adsorb organic materials through hydrogen bonding, serving as a nucleus for further formation of bigger flocs, thus increasing the gravity of the organic particulates. In order to verify this hypothesis, a further test was conducted with humic acid solution.
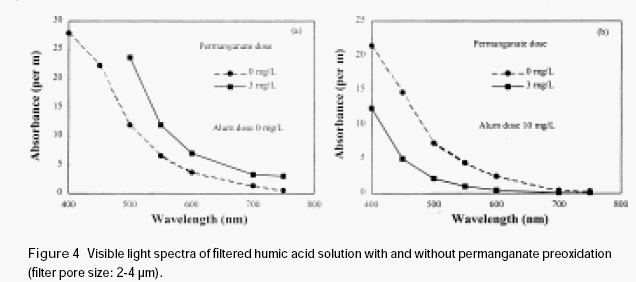
A humic acid solution (5 mg/L) was divided into two parts. One part was directly filtered with a sintered glass filter (pore size 2-3μm); the other part was filtered with the sintered glass filter after it was oxidised by permanganate (3 mg/L). Manganese dioxide was produced
after permanganate preoxidation. The visible light adsorption spectrum of the filtered humic acid solution with and without permanganate preoxidation was shown in Figure 4a. It is shown that the absorbance of the filtered humic acid solution, which was subjected to permanganate preoxidation, increased to a certain extent through the entire visible light range. This is apparently caused by the penetration of manganese dioxide through the filter. It suggests that as soon as manganese dioxide is formed during permanganate preoxidation, it may be adsorbed immediately on the surface of humic acid, forming a highly dispersed stable colloidal solution, and may combine only with difficulty. Thus the newly formed very fine manganese dioxide precipitates may be carried through the filter by humic acid down to the filtered water. It is interesting to note that in the presence of a very small amount of alum (Al2(SO4)3.18 H2O), the absorbance of filtered humic acid solution with permanganate preoxidation is substantially lower than the case without permanganate preoxidation (see fig. 4b). This phenomenon indicates that the manganese dioxide formed in situ has enhanced the removal of humic acid. The manganese dioxide together with the adsorbed humic acid was intercepted on the sintered glass filter. In consequence, it is expected that during the coagulation process, manganese dioxide formed in situ may interact with aquatic organic materials to form an organic-manganese dioxide complex, which may embed very small inorganic and organic particulates and thus increase their sedimentation rate, and also increases their removal efficiency by the filtration process.
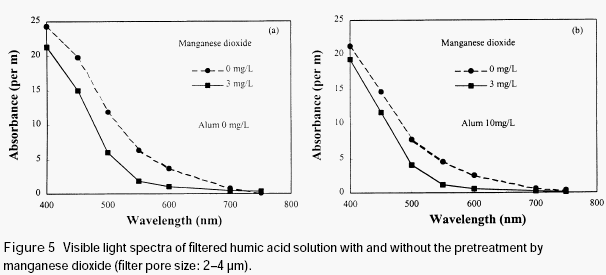
When the humic acid was pretreated with a newly formed manganese dioxide, which was prepared by reduction of permanganate with divalent manganese, the absorbance of the filtered humic acid solution was reduced (see Figure 5), no matter whether or not alum was added before filtration. It is clear that the preformed manganese dioxide also has adsorption ability for humic acid. Since preformed manganese dioxide has relatively bigger size than that formed in situ during permanganate preoxidation, it could be removed by sintered glass filter together with the humic acid adsorbed on its surface, even in the absence of alum.
KMnO4), there was much reduction in the residual turbidity of the settled water. Though further increasing the dose of permanganate caused additional reduction in the residual turbidity, the extent of reduction is relatively small compared to the initial dose of 0.5mg/L. It
is seen from Figure 6(a) that the optimum dose range of PAC was wider than without permanganate preoxidation. This means that the variation of coagulant dose has less impact on the residual turbidity of the settled water. It suggests that the treatment will be more stable when the water is pretreated with permanganate.
Full-scale investigations
Figure 6b shows the results of a full-scale study conducted at Qitaihe water treatment plant.It is clear that the residual turbidity of the settled waters is lower than the train not subjected to permanganate preoxidation. Permanganate preoxidation is very effective in aiding the coagulation of this surface water. It should be noted that the residual turbidities of the fullscale plant operation are higher than those from the jar tests, indicating that the water treatment system needs to be improved. The normal design parameters of conventional water treatment systems are not very suitable for the waters with high organic content, the flocs are not easy to settle because of their very low density.
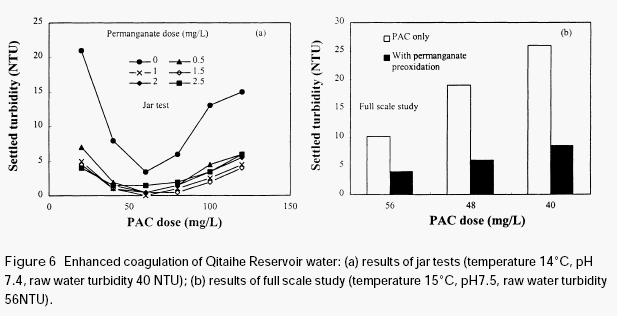
Figure 7(a) shows the variation of the residual turbidity in a full-scale experiment, carried out in Harbin water treatment plant in February 1992. During this period, the turbidity in the raw water was around 20 NTU. The raw water quality is very stable throughout the
experiment (turbidity 19-21 NTU; colour 70-72 and permanganate index 5.23-5.46 mg/L). It is shown in Figure 7(a) that a small amount of permanganate obviously enhanced the coagulation of River Songhua water, with obvious reduction in the settled turbidity (approximately 2-3 NTU reduction). Further increase of permanganate dosage from 0.5mg/L to 1.0 mg/L did not achieve additional reduction in the settled turbidity. After stopping the addition of permanganate, the settled turbidity went up to the original level (around 7 NTU). This demonstrated that permanganate preoxidation is very effective in enhancing the coagulation of River Songhua water. Figure 7(b) shows the results of an experiment conducted in Harbin water treatment plant in November 1992. During the period of conducting the experiment, the raw water turbidity ranged from 12-16 NTU. It was seen that both the quality of the settled and the filtered waters were clearly improved
after the addition of permanganate.
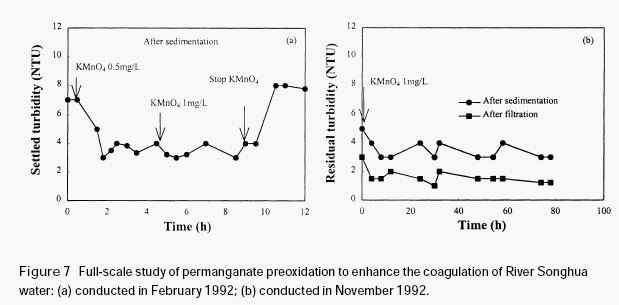
Table 2 shows the quality of the raw water and the treated water in the experiment conducted in February 1992. Since the river was covered with ice during the period of this experiment, the raw water quality was very stable. It can be seen that the quality of filtered water is obviously improved, with obvious reduction in filtered turbidity, colour and permanganate index. The residual manganese (Total Mn) in the filtered water is also lower than the case without permanganate preoxidation. In a previous study (Ma et al., 1997), it was shown that the coagulation pH had a strong influence on the residual manganese in the water in permanganate preoxidation in the filtered water. However, at pH values over 5.5, which is commonly employed in water treatment practice, the residual manganese concentration in the filtered water is very low. The results of this full-scale experiment are consistent with those observed in the previous study.
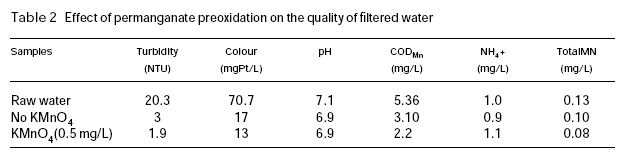
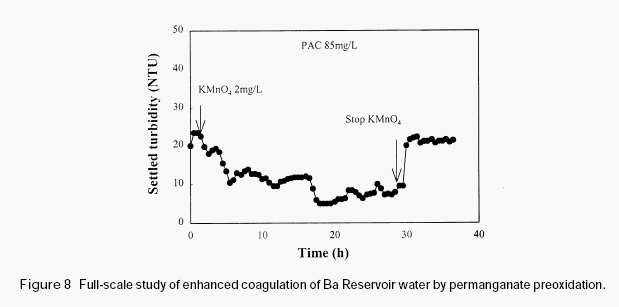
Figure 8 shows the full-scale study conducted at Ba Reservoir water treatment plant. The experiment was conducted in winter, during which the raw water was stable. This water is very difficult to treat and the residual turbidity was normally high when only PAC was used. It can be observed from Figure 8 that after the addition of permanganate (2mg/L) there appeared gradual reduction of the residual turbidity of the settled water. However, after permanganate addition was stopped the residual turbidity of the settled water increased immediately. This experiment further demonstrated the effectiveness of permanganate preoxidation in enhancing the coagulation of the surface waters with high organic content.
Conclusions
Laboratory and full-scale experiments were conducted for enhancing the coagulation of stabilised surface waters with high organic content by permanganate preoxidation. It was indicated that permanganate preoxidation enhanced both the processes of coagulation and filtration of selected surface waters, River Songhua water, Qitaihe Reservoir water and Ba reservoir water. Permanganate preoxidation is especially effective for enhancing the coagulation of very stable surface waters with relatively high organic content. Preliminary comparative
simulation tests demonstrated that permanganate preoxidation is much more effective than prechlorination in enhancing the coagulation of the surface water with high organic content. Experimental results showed that the manganese dioxide produced in situ during permanganate preoxidation played an important role in enhancing the coagulation and filtration processes. The experimental results suggested that manganese dioxide may adsorb naturally occurring organic materials through surface bonding to form bigger particulates, thus increasing the floc density and improving the removal of organic particulates and possibly the inorganic fine particles. Manganese dioxide was shown to adsorb humic acid and enhance its removal by filtration. Full-scale operation results indicated that the residual total manganese concentration in the filtered water can be controlled down to acceptable levels, as long as permanganate is not overdosed.
Acknowledgement
The authors are grateful to the kind support by the National Natural Science Foundation of China (Project Number 59825106). The authors are also grateful for the kind help in water analysis provided by Mr Weihua Bai, Mr Yongxin Jia, and Mr Feng Ji, Ms Quinxia Sun and Ms Baoqin Dong.
References
Becker, W.C., O’Melia, C.R. (1995). The effect of ozone on the coagulation of the turbidity and TOC. Proceedings of the 12th Congress of the International Ozone Assoc, Vol. 1, Lille, France, 15–18 May, 1995.
Chang, S.D., Singer, P.C. (1991). The impact of ozonation on particle stability and the removal of TOC and THM precursors. J Am Water Works Assoc, 83(3), 71–79.
Edwards, M., Benjamin, M.M. (1991). A mechanistic study of ozone-induced particle destabilisation. J Am Water Works Assoc, 83(6), 96–105.
Edwards, M., Boller, M., Benjamin, M.M. (1993). Effect of pre-ozonation on removal of organic matter during water treatment plant operations. Wat Sci & Tech., 27(11), 37–45.
Edzwald, J.K. (1993). Coagulation in drinking water treatment: particles, organics and coagulants. Wat. Sci. Tech., 27(11), 21–35. Gibbs, R.J. (1983). Effect of natural organic coating on the coagulation of particles. Envir Sci & Technol, 17,237–240.
Glaze, W.H., Weinberg, H.S., and Cavanagh, J.E. (1993). Evaluating the formation of brominated DBPs during ozonation. J Am Water Works Assoc, 85(1), 96–103.
Jekel, M.R. (1983). The benefit of ozone treatment prior to flocculating processes. Ozone Sci Engng, 5(1),21–35.
Jekel, M.R. (1986). The stabilization of dispersed mineral particles by adsorption of humic substance. Water Research, 20(12), 1543–1554.
Lefebvre, E., Paillard, H., Legube, B. (1990). The effect of ozonation on the removal of organics by coagulation/flocculation. Ozone Sci Engng, 12(3), 295–313.
Li, G.B. and Ma, J. (1992). Removal of mutagens from polluted water and control of mutagenic activity by permanganate pretreatment. Water & Wastewater Engineering, 18(2), 15–18.
Ma, J. and Graham, N. (1996). Controlling the formation of chloroform by permanganate preoxidation –destruction of precursors. J Water SRT-Aqua, 45(6), 308–315.
Ma, J. and Li, G.B. (1991). Organic pollutants removal by permanganate oxidation. In Water and Wastewater Research. Edited by Xushi Yan, China Architecture Industry Press, 12–28 (In Chinese).
Ma, J. and Li, G.B. (1993). Laboratory and full-scale plant studies of permanganate oxidation as an aid in the coagulation. Wat Sci & Technol, 27(11), 47–54.
Ma, J. and Li, G.B. (1994). Control of chlorophenol formation in chlorine-disinfection process by permanganate preoxidation. Proceedings of the International Conference and Exhibition on Water and
Wastewater ‘94, Beijing, China, July 1994, International Academic Publishers, 201–208.Ma, J., Li, G. and Graham, N.J.D. (1994a). Efficiency and mechanism of acrylamide removal by permanganate oxidation. J Water SRT-Aqua, 43(6), 287–195.
Ma, J., Li, G.B. and Graham, N.J.D. (1994b). Removal of acrylamide by permanganate oxidation. Water SRT-Aqua, 43(6), 287–295.
Ma, J., Graham, N.J.D., and LI, G.B. (1997). Effectiveness of permanganate preoxidation in enhancing the coagulation of surface waters – Laboratory studies. J Water SRT-Aqua, 46(1), 1–11.
Narkis, N. and Rebhun, M. (1975). The mechanism of flocculation processes in the presence of humic substances. J Am Water Works Assoc, 67, 101–108.
O’Melia, C.R. (1987). Particle-particle interaction. In Aquatic Surface Chemistry (W. Stumm Eds.), Wiley Interscience, New York.
Petrusevski, P., van Breemen, A.N., Alaerts, G.J. (1995). Optimisation of coagulation conditions for direct filtration of impounded surface water. J Water SRT-Aqua, 44(2), 93–102.
Preis, S.V., Siirde, E.K., Pyldoya, L.I. (1991). Role of preozonation in treating lake Ylemiste water. Soviet J Water Chem Technol, 13(8), 81–84 (Abs.).
Reckhow, D.A., Singer, P.C. (1984). The removal of organic halide precursors by preozonation and alum coagulation. J Am Water works Assoc, 76(4), 151–157.
Singer, P.C., Bochardt, J.H., Colthurst, J.M. (1980). The effects of permanganate pretreatment on trihalomethane formation in drinking water. J Am Water Works Assoc, 72(10), 573–578.
Tobiason, J.E., Edzwald, J.K., Reckhow, D.A., Switzenbaum, M.S. (1993). Effect of Pre-ozonation on the organics removal by in-line direct filtration. Wat. Sci. Tech., 27(11), 81–90.
Yao, C. and Yan, X. (1989). Removal of turbidity and colour by alum. Shanghai Water and Wastewater, 2,1–5.
论文搜索
月热点论文
论文投稿
很多时候您的文章总是无缘变成铅字。研究做到关键时,试验有了起色时,是不是想和同行探讨一下,工作中有了心得,您是不是很想与人分享,那么不要只是默默工作了,写下来吧!投稿时,请以附件形式发至 paper@h2o-china.com ,请注明论文投稿。一旦采用,我们会为您增加100枚金币。








